Abstract
Sialic acid, primarily occurring as N-Acetylneuraminic Acid (NANA or Neu5Ac), is a critical carbohydrate derivative utilized extensively in infant formula, dietary supplements, and cosmetic formulations. For B2B procurement managers and R&D professionals, navigating the complexities of the sialic acid raw material market requires a deep understanding of molecular purity, production methodologies, and regulatory compliance. This guide provides a technical overview of sialic acid powder, evaluating its functional applications and the essential criteria for selecting a reliable N-Acetylneuraminic Acid manufacturer to ensure supply chain stability and product efficacy.

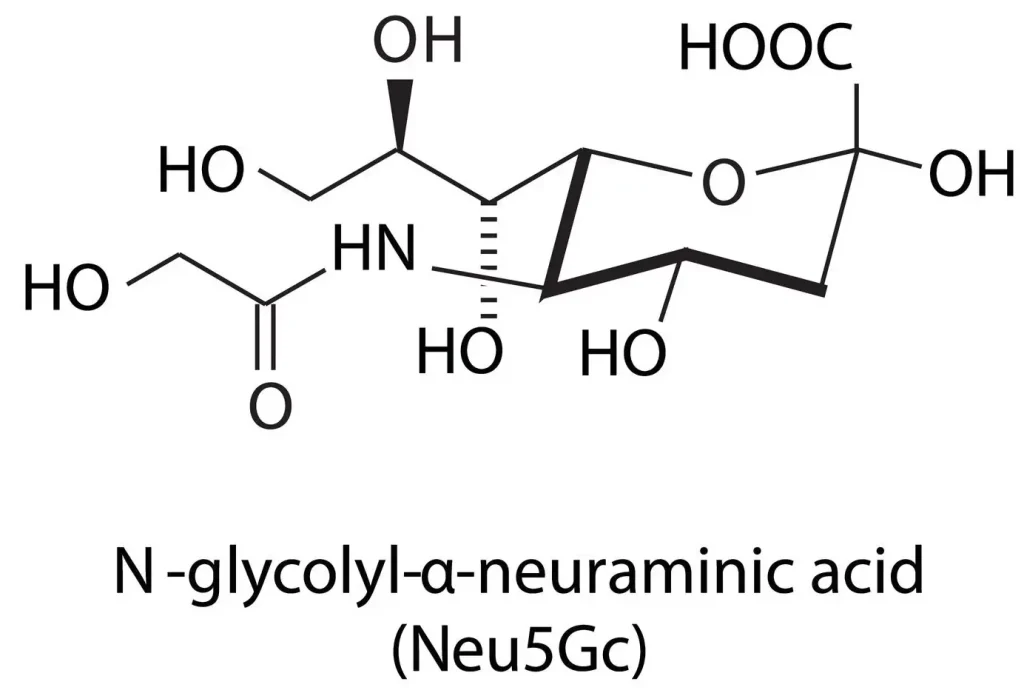
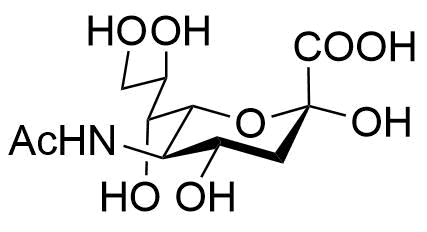
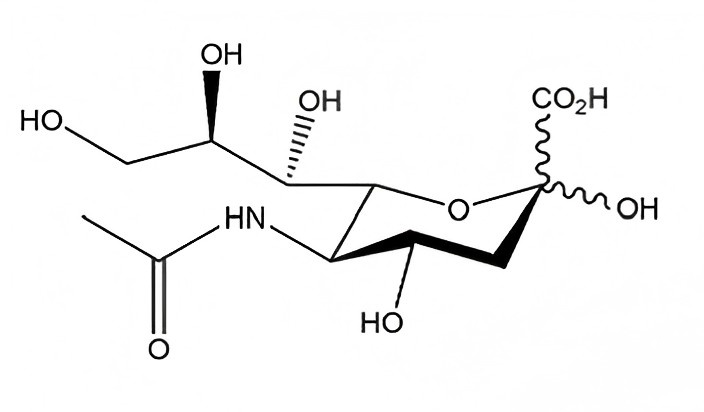
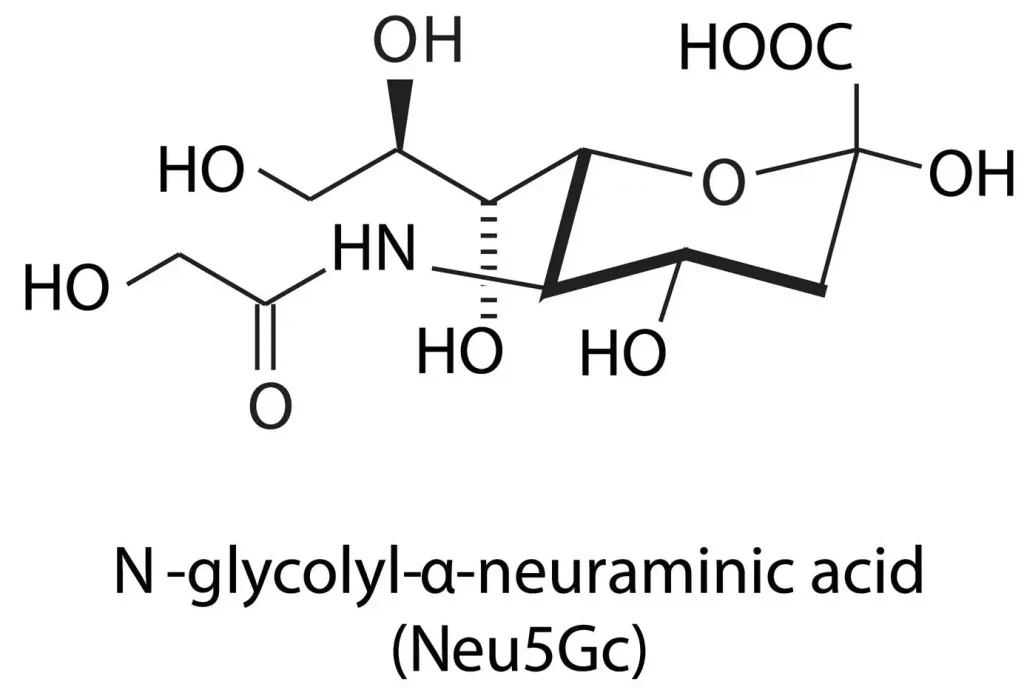
The Molecular Foundation: N-Acetylneuraminic Acid vs. Sialic Acid
In technical procurement, the terms “Sialic Acid” and “N-Acetylneuraminic Acid” (NANA) are often used interchangeably, though it is vital to recognize their relationship. Sialic acid represents a family of over 50 neuraminic acid derivatives. However, in the commercial raw material sector, N-Acetylneuraminic Acid is the most prevalent and biologically active form.
When sourcing sialic acid powder, professionals must verify the CAS number (131-48-6) to ensure the material matches the specific isomer required for human applications. As a foundational component of glycoconjugates, NANA plays a pivotal role in cellular recognition and neurological development, making it a high-value ingredient for specialized product lines.
Industrial Production: Fermentation vs. Extraction
The shift in the global supply chain has moved toward sustainable biomanufacturing. Historically, sialic acid was sourced from animal by-products or bird’s nests, which presented challenges in scale and purity.
Today, a leading N-Acetylneuraminic Acid manufacturer typically employs advanced microbial fermentation. This synthetic biology approach allows for:
-
Higher Purity: Achieving concentrations of 98% or higher, free from animal-derived pathogens.
-
Sustainability: A more environmentally conscious footprint compared to traditional extraction.
-
Consistency: Batch-to-batch uniformity that meets the rigorous standards of the pharmaceutical and nutraceutical industries.
Critical Quality Indices for Sialic Acid Raw Material
When evaluating sialic acid raw material, the technical data sheet (TDS) should be scrutinized beyond simple purity percentages. Key metrics for industrial-grade supply include:
-
Heavy Metal Limits: Strict adherence to Lead (Pb), Arsenic (As), and Mercury (Hg) limits, typically <1ppm or <0.1ppm depending on the application.
-
Microbiological Profile: For use in infant formula or food supplements, total plate counts and the absence of pathogens (E. coli, Salmonella) are non-negotiable.
-
Stability and Solubility: The powder must demonstrate excellent water solubility and thermal stability to survive various manufacturing processes, such as spray drying or pasteurization.
Strategic Procurement: Identifying a Reliable Manufacturer
Choosing a sialic acid manufacturer involves more than comparing price per kilogram. For long-term viability, B2B partners must evaluate:
-
Compliance and Certification: Ensure the facility holds international certifications such as ISO 9001, ISO 22000, and GMP. For food-grade applications, Halal and Kosher certifications are often essential for market entry.
-
R&D Support: A manufacturer with robust R&D capacity can provide customized particle sizes or formulations tailored to specific delivery systems (e.g., capsules vs. functional beverages).
-
Traceability: Full transparency in the supply chain, from the fermentation substrate to the final packaging, is required for modern regulatory audits.
Conclusion
The demand for high-quality N-Acetylneuraminic Acid powder continues to grow as its benefits for cognitive health and immune support become more widely recognized. For businesses, the priority lies in securing a transparent, scientifically-backed supply of sialic acid powder that complies with global safety standards. By focusing on technical purity and manufacturing excellence, procurement teams can successfully integrate this potent ingredient into their product portfolios with confidence.
FAQ
Q: What is the standard shelf life for bulk N-Acetylneuraminic Acid powder? A: Most manufacturers provide a 24-month shelf life when stored in a cool, dry place in original sealed packaging. However, stability testing reports should be requested for specific environmental conditions.
Q: Is your sialic acid raw material compatible with vegan formulations? A: Yes, if produced via microbial fermentation, the resulting powder is typically 100% vegan and animal-free, making it suitable for clean-label product positioning.
Q: Why do prices vary significantly between different sialic acid powder suppliers? A: Pricing is often dictated by the production method (fermentation vs. extraction), the level of purification, and the rigor of the quality control process. Materials intended for pharmaceutical use or infant formula require higher compliance standards, which is reflected in the cost.
Q: How does a manufacturer ensure the absence of Neu5Gc in NANA? A: High-end manufacturers utilize precise fermentation strains and HPLC (High-Performance Liquid Chromatography) testing to ensure that the product is pure Neu5Ac (NANA) and does not contain Neu5Gc, which is an important distinction for human-grade supplements.